ACS Materials Letters Paper Published
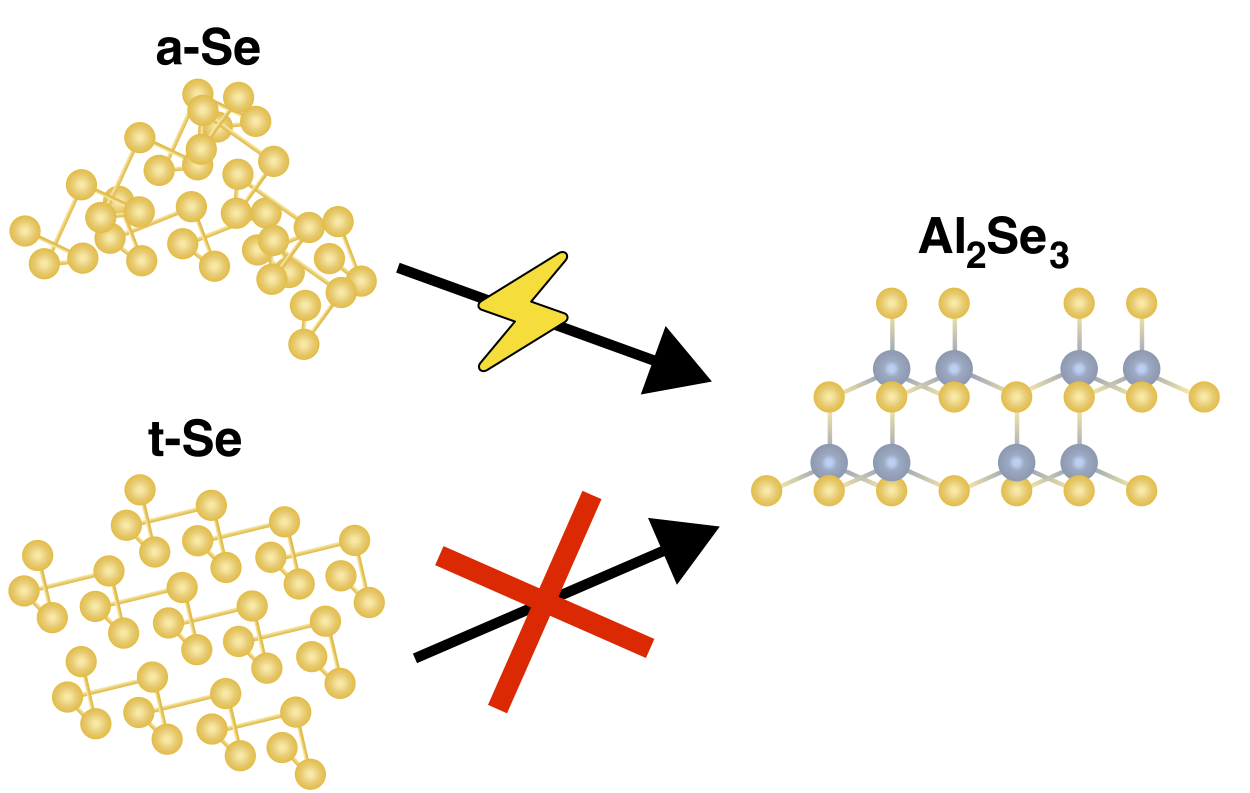
We just published a fundamental investigation of selenium structure at different length scales and how it affects the electrochemical reactions achieved in aluminium-selenium batteries. To achieve a full 6-electron capacity of selenium electrodes in aluminium batteries, it is imperative that the Se(0) to Se(–II) is attainable as well as the Se(0) to Se(IV) reactions. This paper documents the viability of the selenium to aluminium selenide electrochemical reduction in glassy selenium, which is seldom observed with crystalline trigonal selenium. This paper challenges the naive assumption that aluminium batteries using sulfur and selenium cathodes would operate in the same way. While both are chalcogens, the bonding topologies of elemental selenium and sulfur are quite different in their most stable forms (trigonal Se chains vs. 8-membered S rings) and this leads to differences in their electrochemical performances.