Aluminium-chalcogen Batteries
Our group has done work to pursue the uncover the details of Aluminium-chalcogen energy storage.
Our group has done work to pursue the uncover the details of Aluminium-chalcogen energy storage.
Our group has worked to develop understanding in aluminium-chalcogen batteries, primarily through NMR characterisation. We have worked with both aluminium-sulfur, and aluminium-selenium systems and we strive to identify their common features and their distinct characteristics. We probe the electrochemical reaction products in these systems to attain an understanding of their electrochemical properties and reversibility.
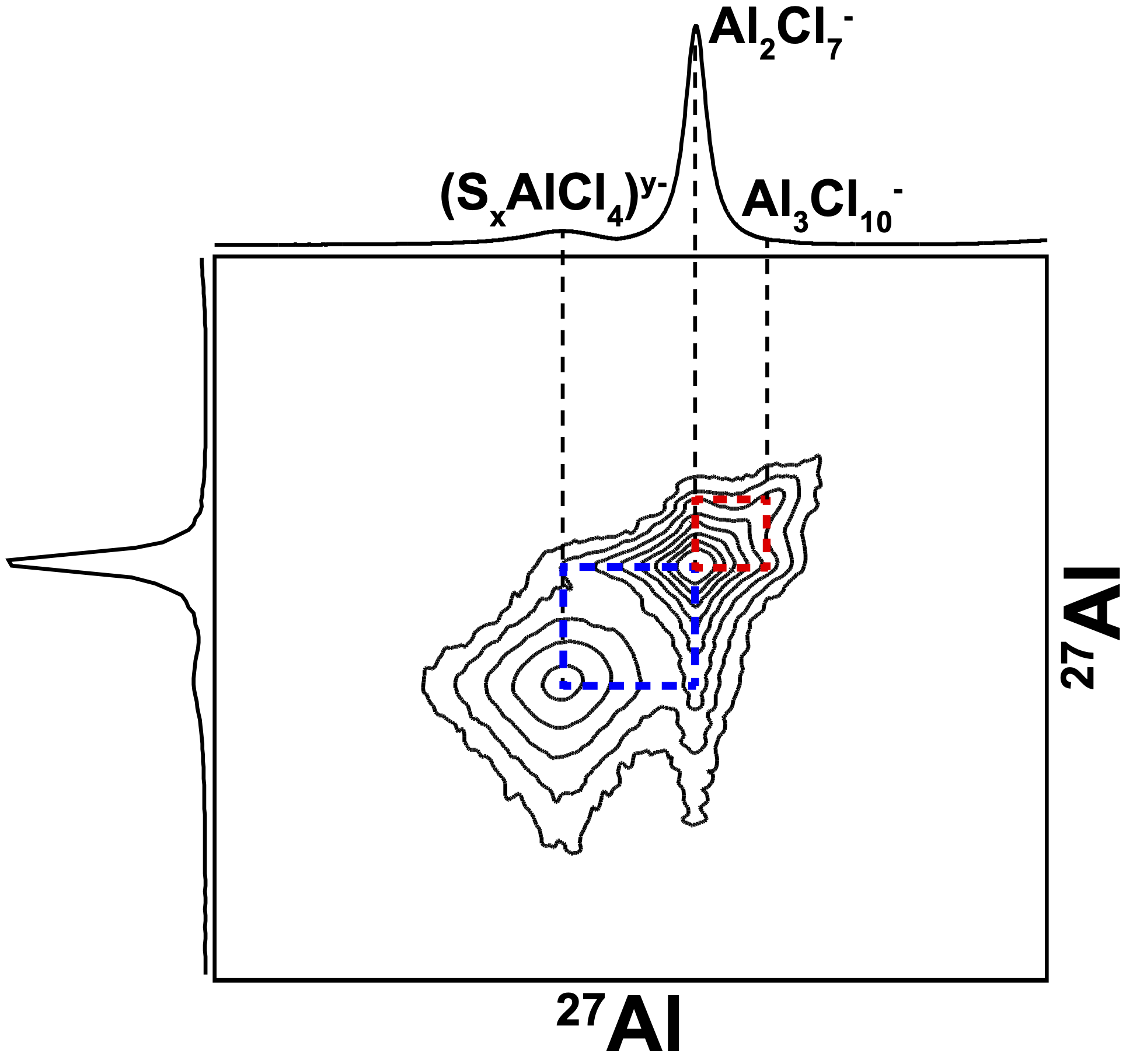
In the sulfur system, we use 27Al NMR to distinguish the solid and liquid reaction products and how they interact. We additionally highlight the different aluminium sulfide polymorphs present in commercially available powders and how that compares to electrochemically generated Al2S3. The paper we published in ACS Chemistry of Materials can be found at this link.
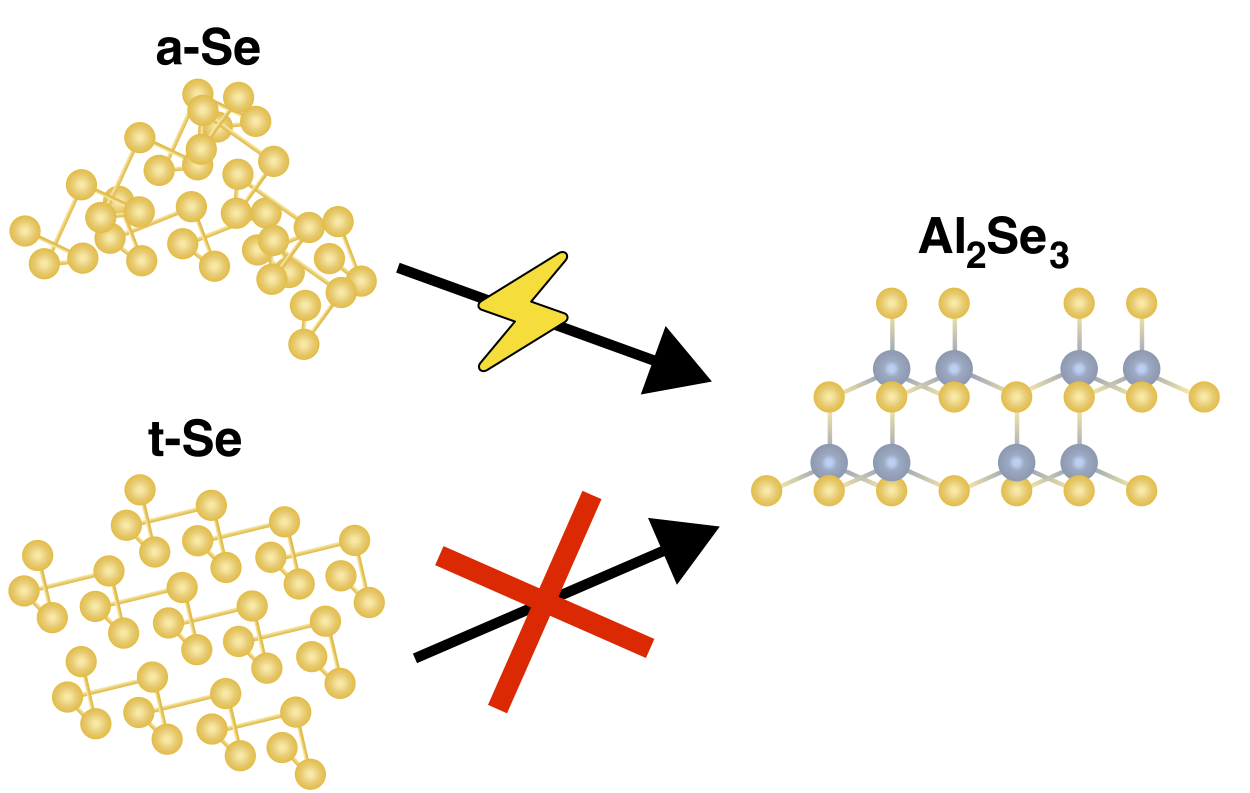
Similarly, we were able to probe the local structure of selenium through solid-state 77Se NMR, where we showed that upon melting, semi-conducting grey selenium (hexagonal structure forming trigonal helices) retains its bonding topology at the molecular level, but the inter-chain interactions of amorphous selenium yield a higher propensity for the electrochemical discharge reaction of Se to Al2Se3. The crystalline form is scarcely able to undergo electrochemical discharge, however, the selenium formed after the facile oxidation reactions to SeCl4 is less crystalline, and therefore more able to perform the Se(0) to Se(–II) reaction. It is important to note that while sulfur and selenium are both elemental chalcogens, sulfur forms 8-membered rings, whereas generally selenium in used in its trigonal helix structure (the 8-membered ring form of selenium, red selenium, is a metastable state). Due to their differences, the electrochemical reactions they undergo can be different. This study can be read in full here.