Additives for Lithium metal Battery Electrolytes
Using additives can improve the performance of lithium metal batteries. We seek to understand the chemical species leading to this improvement.
Using additives can improve the performance of lithium metal batteries. We seek to understand the chemical species leading to this improvement.
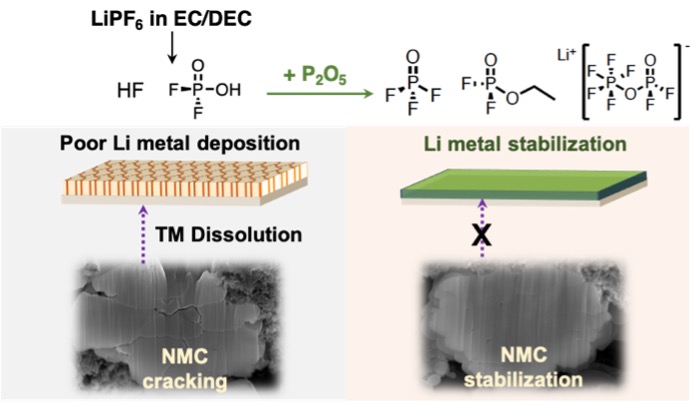
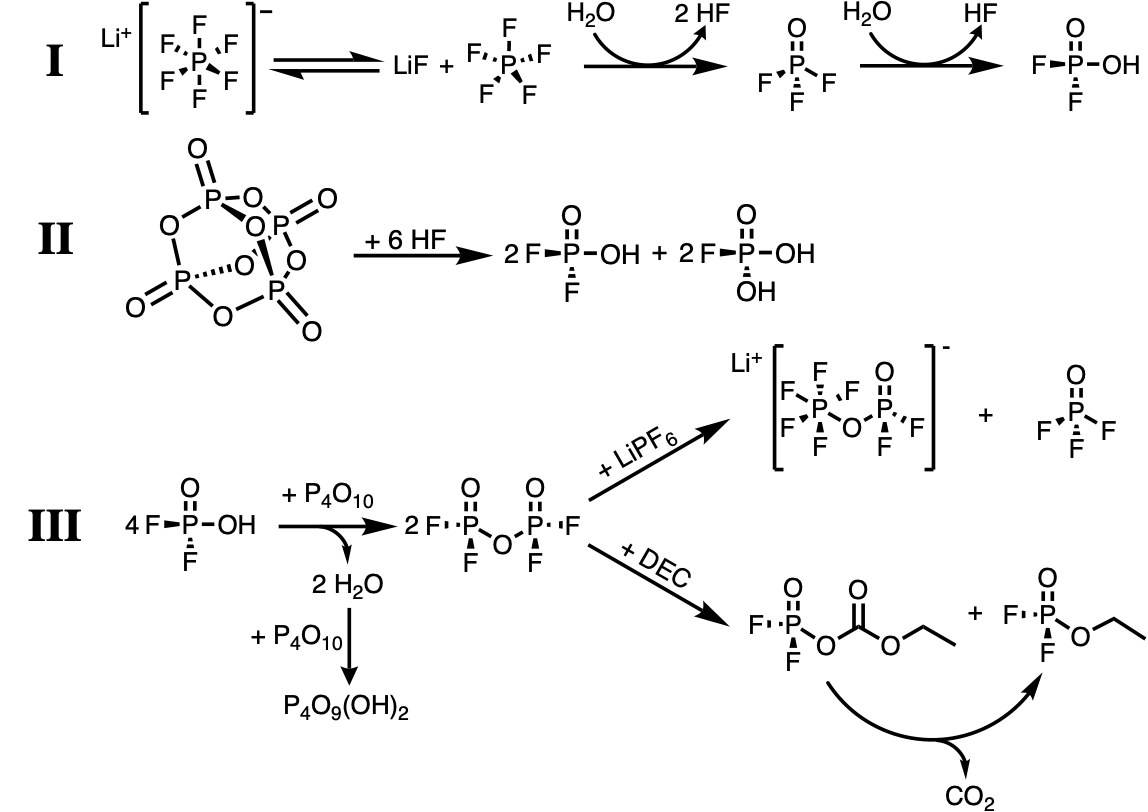
Lithium metal batteries using liquid electrolytes often suffer from dendritic growths leading to short circuiting of the cells. In addition liquid carbonate electrolytes for lithium batteries are prone to decomposition, forming HF. Addition of P2O5 scavenges HF and residual water which could react forming further HF, while also producing a stable and favourable SEI layer on the lithium surface, enabling smooth plating and stripping. We compliment this extraordinary performance with a highly quantitative, liquid-state 19F and 31P NMR, complimented by 1H and 13C NMR to both characterise the species responsible for the performance enhancement and to develop a reaction scheme of how these species might be formed.
This work represents a crucial step forward in the field of Li-metal batteries, enabling existing processing and materials to be used while avoiding the pernicious reactions that are renowned for destroying Li-metal cell performance, on both the lithium metal and on the cathode materials, which are susceptible to transition metal leaching in the presence of HF. Our proposal of the mechanistic pathway for acid and water scavenging may also highlight to other researchers alternative materials that could be used as liquid electrolyte additives for Li-metal batteries.
The paper can be accessed here.