Organic Electrodes for Multivalent Batteries
We strive to characterise organic batteries to help target optimal structures for high performing electrodes in different battery chemistries.
We strive to characterise organic batteries to help target optimal structures for high performing electrodes in different battery chemistries.
In collaboration with the John Group at CCNY we have been developing understanding of electrochemical ion complexation to organic molecules, with a specific focus on quinone-type organics.
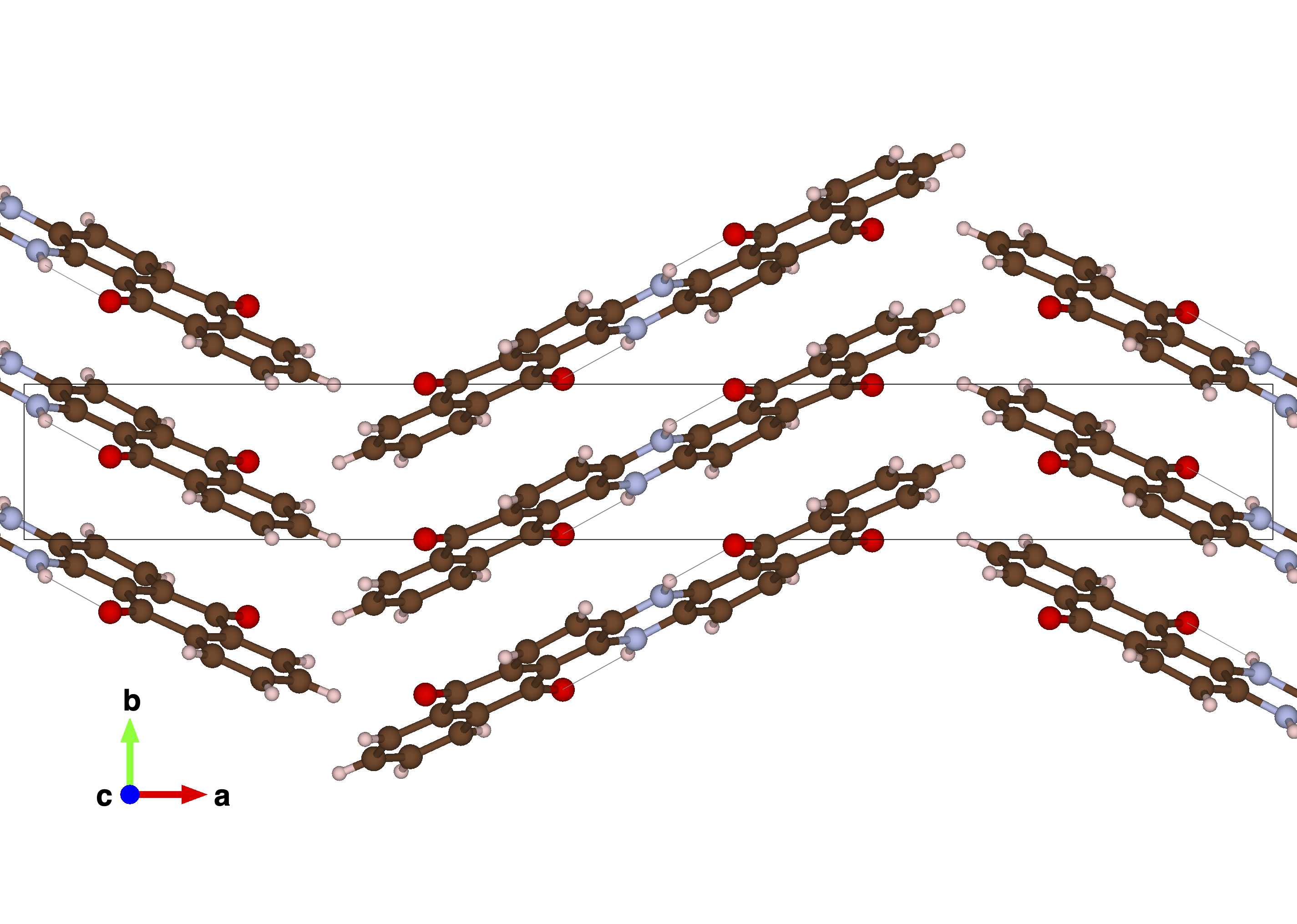
Our first paper on the topic, published August 2022, highlighted indanthrone quinone (INDQ) as a high-performance cathode material for Al-organic batteries and studied the electronic and ionic charge storage mechanisms in depth. Using a rigorous solid-state NMR methodology, this investigation established the molecular changes on the organic in tandem with the nature of the electroactive ions and, in concert with the electrochemical analyses, determined a feasible explanation for the curious generation of aluminium-containing cationic species in an electrolyte natively containing only anionic aluminium species.
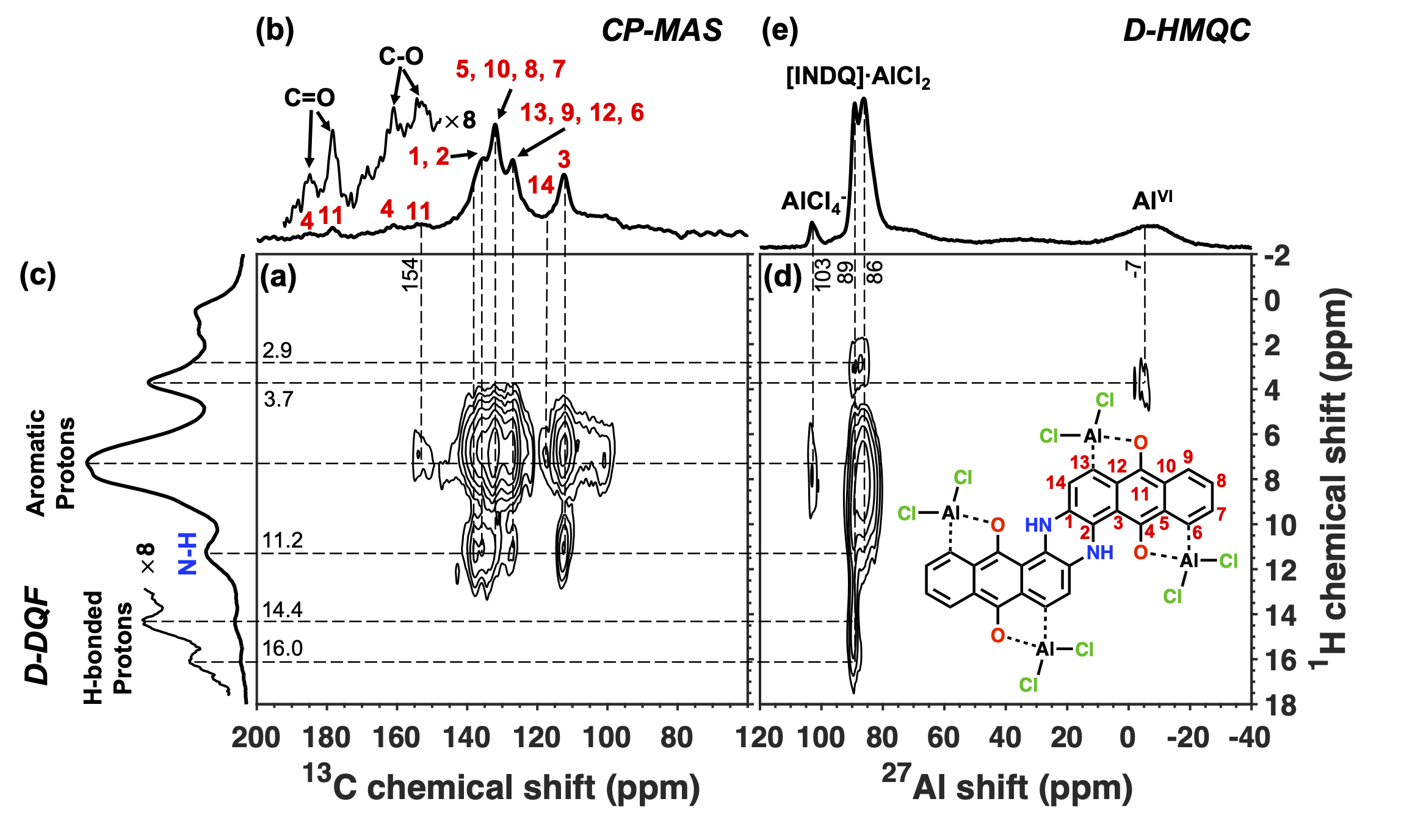
Our second publication on aluminium-quinone battery charge storage mechanisms, published in the Journal of Magnetic Resonance in Januray 2023, investigated the impact of electrolyte speciation on resultant ionic charge storage mechanisms.
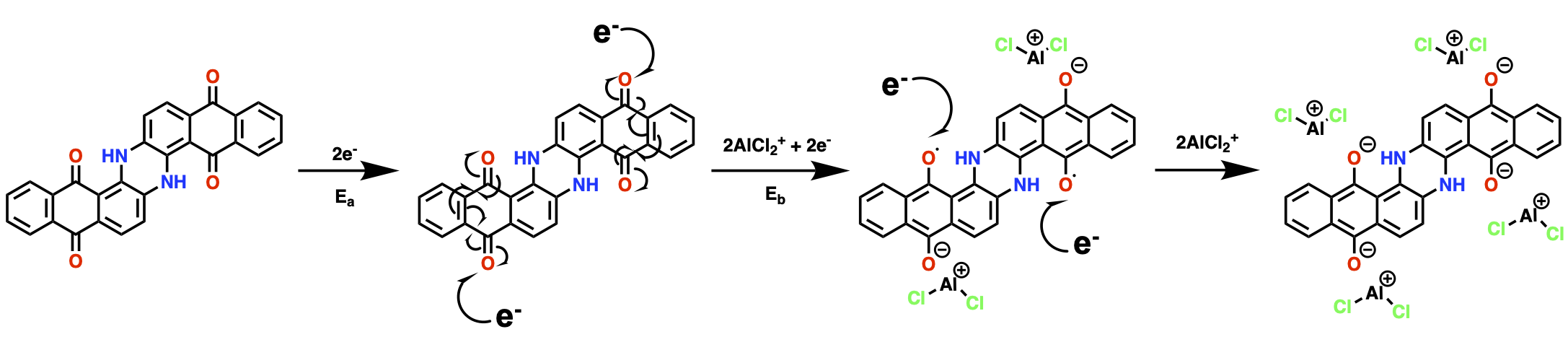
Here, we used 1,5-dichloroanthraquinone (DCQ) as our electrode material This work was ideated by a curiosity from our previous work in Al-organic batteries where we found that in electrolytes with only anionic aluminium ions, the organic electrodes are charge compensated by cationic aluminium compounds! As a result, we set out to determine the possible mechanisms for producing these cations in-situ, and what the mechanisms are when cationic aluminium compounds are already present in addition to the anions.
We further set out to interpret the physical origins of specific quadrupolar lineshapes observed in the solid-state NMR spectra of discharged electrodes. Each of the three electrolytes used yielded different 27Al spectra, some with multiple environments where only one was expected. This we studied through DFT calculations of complexed ions in a range of different binding configurations to understand the trends between the aluminium binding environment and the NMR quadrupolar parameters. We also used DFT to determine the viability of ion generation pathways using thermochemical calculations, which we validated experimentally.
Overall, we concluded that there are competing mechanisms occurring, where some will utilise a combination of ions in the electrolyte until one species is consumed, whereupon the other, less favoured reactions will begin to dominate. In addition, we observe that the choice of organic ligating component (L = amide, urea, etc.) in ionic liquid analogues will change the resultant binding environments in aluminum-organic batteries. This was indicated by the change in interlayer quinone spacing caused by the charge-carrying electroactive ions which contain these organic species (e.g., AlCl2L2+), confirming their role in the ionic charge storage mechanism.
One other goal is to understand from the basic level, what chemical and electronic effects substituents have on the fused aromatic ring system of anthraquinone and how we can tune these organic molecules for optimal performance. Our aim is to develop this fundamental understanding before oligomerising/polymerising these molecules to mitigate issues due to solubility.
In currently working aluminum battery electrolytes, it appears that the majority of charged species being stored are not necessarily multivalent. Unfortunately due to issues of the aluminum metal anode in aqueous electrolytes (another project of interest in our lab), attaining the full 3+ charge from aluminum is difficult to achieve from an aluminum-metal battery.
We have studied anthraquinone-based cathodes extensively in aluminum-metal battery systems, and we are further investigating the generalisability to zinc-aqueous batteries. The zinc-organic battery investigation is underway as an undergrad project in Spring 2022.